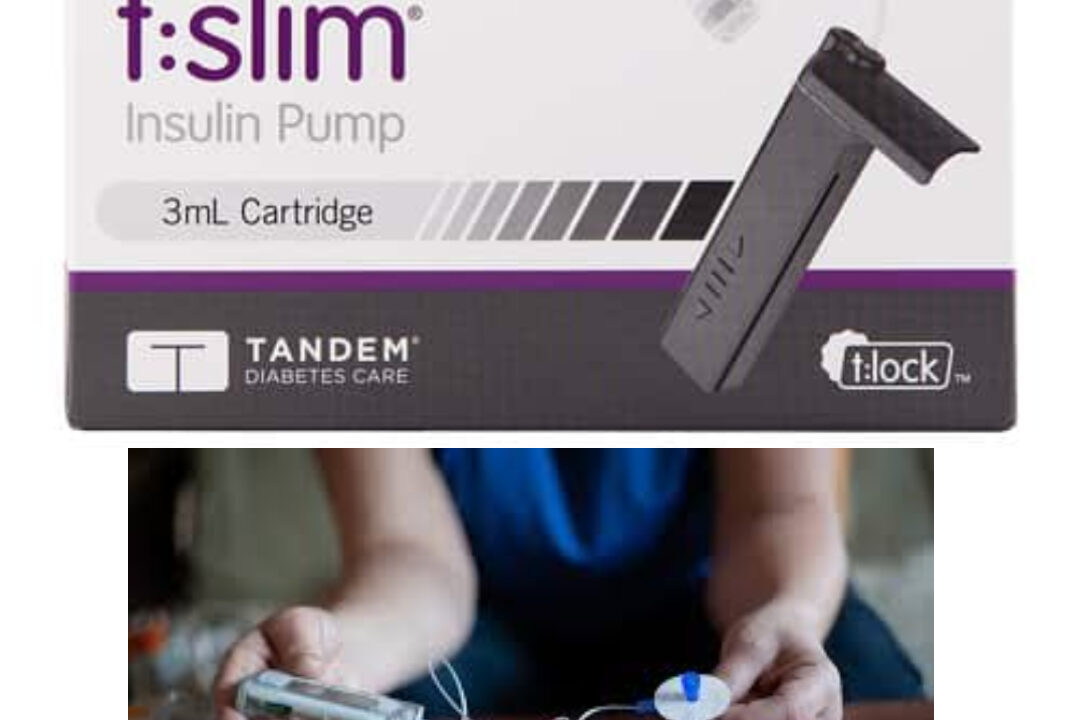
There are some pros and cons to the t slim insulin pump. The first is that it costs more than other insulin pumps. It also requires a prescription from a doctor. A second drawback is that it's intended for single patient use. Finally, it's not interoperable with other diabetes management devices. Weigh these cons before you make your final decision. Read on for more information about this insulin pump. Weigh the pros and cons and decide which is right for you.
Costs more than other insulin pumps
The cost of Humalog, a fast-acting insulin, has skyrocketed since its introduction in 1996 by Eli Lilly. A vial of the drug then cost $21, but today's price is nearly ten times higher. Despite this, insulin manufacturers are able to charge the high prices because they are able to patent incremental changes to their products. These patents make it difficult for competitors to enter the market and provide a cheaper alternative. The high cost of insulin is due to several factors, including the increasing complexity of the supply chain.
Insurance companies don't cover the full cost of insulin pumps, and there are strict guidelines before insurers will pay for the devices. In addition, the pumps may cause infection at the catheter site. Moreover, if the insulin pump catheter is accidentally removed, it can lead to diabetic ketoacidosis, a life-threatening complication. In addition to these costs, insulin pumps may require hospitalization for training. This is why it's best to check with your doctor and insurance company before deciding to buy one. However, if cost is the biggest concern, consider a different approach.
In a study, the costs of continuous insulin pump therapy were compared to MDI. This study employed the NCDR, a nine-year observational panel from national health data registers, and socioeconomic data registers. The results are shown in Figure 2B. The results show that pumps were more expensive than MDI, but the cost increments were reduced. The costs were higher for women, but the results were less stark.
The research also revealed that insulin pens have significantly lower healthcare expenses than syringes. Pens, on the other hand, come in packs of five. A box of five Humalog KwikPens or five authorized generics can cost upwards of $300. However, some insurance policies cover the cost of insulin pens, so you can save money on healthcare overall by switching to these. So, you can now choose between insulin pens and insulin pumps.
A few pros and cons of insulin pens include: their lack of portability and higher costs. Pens are also more convenient, but their cost is higher. You can only use one type of insulin per month, and you need to prime the needle every two weeks or so. Another disadvantage of insulin pens is that they may not work with multiple types of insulin. Depending on your situation, you may need more than one type of insulin per month.
Is intended for single patient use
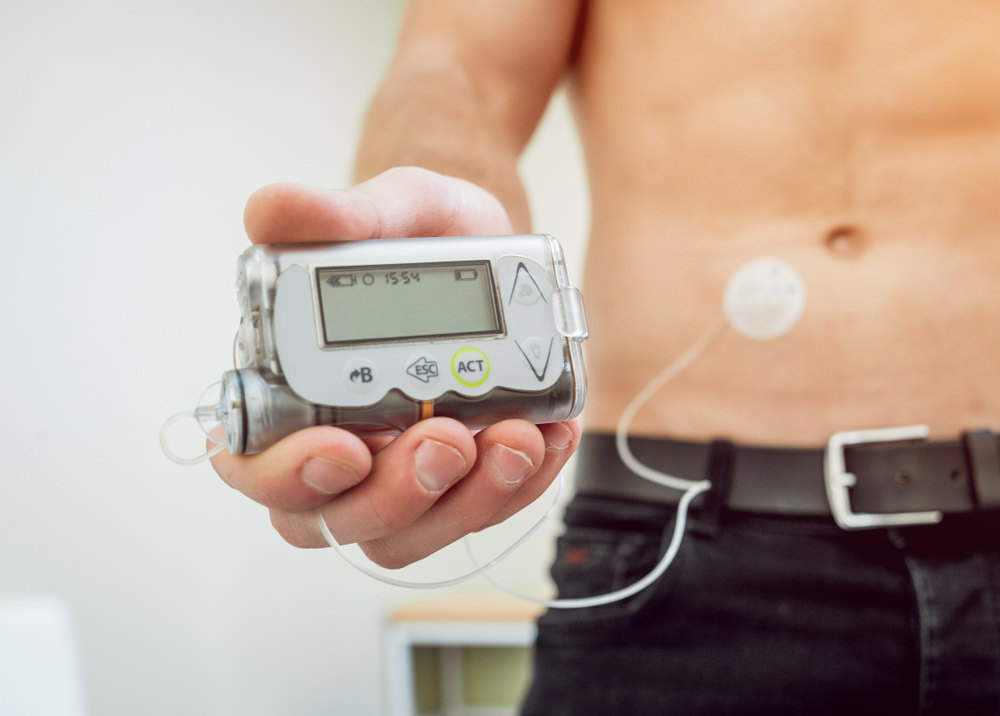
There are some advantages to using single-use items over reusable ones. Because they are only used on one patient and should not be reprocessed, they reduce the risk of cross-contamination. The disadvantages include not being suitable for heat-tolerant cleaning and usually being made of cheap metals. They are also not designed to be sterilized or disinfected. Therefore, single-patient-use items should only be used once.
Most medical devices are designed for single-use. Most of them do not require complex reprocessing and cleaning procedures. Most manufacturers of single-use devices provide written instructions for cleaning, decontamination, and disposal of their products. The guidelines can help healthcare providers and professional staff understand the necessary steps to maintain their sterilization and safety during the single-patient-use process. The reprocessing guidelines can be referred to in the product's manufacturer's website.
The FDA has recently announced that it is retiring the term “single-patient use” and instead is creating a new term to describe package types. Single-patient-use containers contain multiple doses of injectable medical products. They should be labeled appropriately so that they do not pass the risk of bacterial infection between patients. This term should also be used in combination products and drug products that are designed for single-patient use.
Medical devices that are designed for single-use are the best option for preventing cross-contamination. Reprocessing single-use items can compromise their effectiveness, safety, or efficiency and could put patients at risk. As such, it is important to avoid reprocessing devices to prevent these risks. To avoid this, manufacturers should clearly mark single-use medical devices with a “do not reprocess” symbol.
Is interoperable with other diabetes management devices

Interoperability is the term used to describe a device's ability to exchange information with other devices in a diabetes management system. This functionality is especially important when a person with diabetes needs to monitor blood glucose levels over a longer period of time. Interoperability helps patients by making it easier to share their diabetes data and track their progress. In addition, it allows health care providers and patients to have better access to the latest innovations in diabetes management.
While integrating diabetes data has long been a challenge, recent scientific advancements have improved our understanding of how to share information and make new technologies easier to use. Although diabetes management devices are often capable of sharing data between themselves, they are not generally interoperable. Lack of interoperability restricts the benefits of today's advanced diabetes technologies and prevents the development of new ones. This article examines four scenarios in which enhanced interoperability could benefit diabetes management.
Today's diabetes devices are increasingly becoming interoperable with one another, and this means that a person can use the same device with multiple glucose monitors, insulin pumps, and other devices. Interoperability will allow caregivers to access data from each device in a single, central location. By allowing these devices to communicate with each other, patients will have a greater safety net. But how can this be done?
One important step to achieving interoperability is to create a standard for the communication protocols. This would enable diabetes management devices to work with other diabetes management devices that have different interfaces. Ultimately, interoperability standards could create technological solutions for regulatory hurdles. In the meantime, it's essential to identify the responsible party of these devices. But what should a diabetic patient do if the device cannot connect to the rest of his or her equipment?
One example of interoperability is a glycemic control software, which is connected to an integrated continuous glucose monitor (ICGM) and an insulin pump. Together, the devices create a closed loop system that allows diabetic patients to tailor their diabetes treatment to their needs. It's also called an automated insulin dosing system. Interoperability is defined by the FDA as the ability for one product to share information with another, whether that information is for displaying, storing, analyzing, or controlling another product.