
You may have heard of the Omnipod pump, but what exactly is it? This tubeless automated insulin delivery system is covered by Medicare Part D, is waterproof, and integrates with a Dexcom G6. In this article, we'll discuss how the Omnipod pump works and answer some of the most frequently asked questions about the device. You'll also learn about its benefits, including how it can help you manage your diabetes.
Table of Contents
Omnipod pump is a tubeless automated insulin delivery system
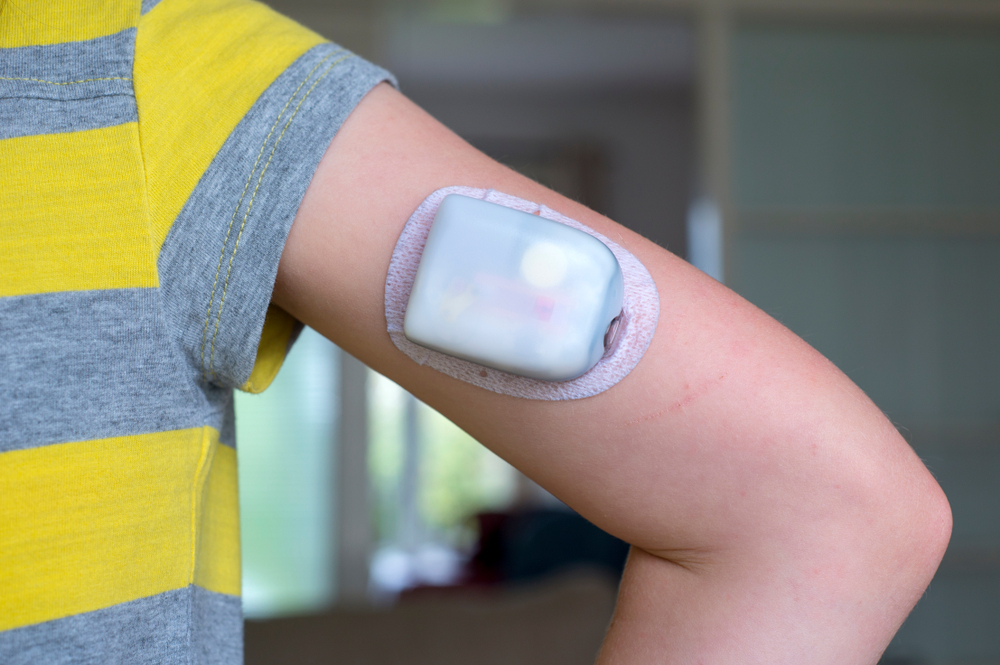
The Omnipod pump is a tubeless automated system that uses a continuous glucose monitor and a tubeless pod. It is equipped with SmartAdjust technology to automatically adjust the amount of insulin you receive in response to your glucose levels. The system also features a mobile app with a SmartBolus Calculator and a controller that comes free with your first prescription. One of the most important features of the Omnipod is the SmartAdjust technology, which is designed to receive trend data from a Dexcom G6 continuous glucose monitor every five minutes. The algorithm can then increase insulin delivery using a personalized target glucose level. This feature is designed to help you avoid dangerously high glucose levels and keep your blood sugar level within the proper range.
The Omnipod 5 is the first tubeless automated insulin delivery system to be integrated with a Dexcom G6 continuous glucose monitoring system. It works in conjunction with the device and uses a controller algorithm to automatically adjust insulin delivery to keep blood glucose levels stable. It is also approved as part of a management protocol, which means you don't have to worry about taking multiple injections every day. The Omnipod pump is also water-resistant, which means you won't get sick because you don't have to worry about spills and leakage.
The Omnipod 5 is the latest product to come from Insulet Corporation, a global leader in tubeless insulin delivery systems. This tubeless pump integrates with the Dexcom G6 continuous glucose monitor and a compatible smartphone to help you monitor your blood sugar levels. The Omnipod 5 will be available to people in limited markets early next year and more broadly in September 2020. It is a new way to manage diabetes.
The Omnipod 5 closed loop automated insulin delivery system is now available in the United States after being cleared by the FDA. This system can be controlled with a smartphone, and is now the fourth commercially available AID system in the U.S. for people with type 1 diabetes. Although it is a small device, it is effective in providing continuous nonstop insulin delivery. The Omnipod pump is also capable of delivering other subcutaneous drugs.
It is covered by Medicare Part D
You may be wondering whether the Omnipod insulin pump is covered by Medicare Part D. While it is not listed on the CMS website, the Omnipod is an approved insulin pump under the Medicare Part D program. However, Medicare Part D has a special process to allow it to be prescribed. The Omnipod pump is covered by Medicare Part D if you have a PDP. You can find out if the Omnipod pump is covered by Medicare Part D by contacting your plan's provider.
The FDA cleared the Omnipod pump in December after a review that revealed it meets the stringent requirements for the Medicare program. In addition to ensuring that the pump is covered by Medicare, the approval also allows it to be covered by Medicaid. Fortunately, this is now the case. While it took three years for CMS to cover the pump, it has finally been approved. The decision may make the Omnipod pump covered by Medicare Part D for more people.
The FDA's approval may lead to the expansion of Omnipod pump coverage to private health insurers and state-run Medicaid programs. This could benefit the 450,000+ people living with type 1 diabetes in the US. With the new policy, type 2 diabetes patients could also receive coverage. For now, there is no definite plan for when Omnipod pump will be covered by Medicare Part D. Its reimbursement depends on how quickly the FDA approves the pump.
Since Omnipod is covered by Medicare Part D, it is important to know the specific coverage details before purchasing the device. The cost of an Omnipod pump varies by carrier. A five-pack can last up to 72 hours. The cost of an Omnipod is roughly $250 to $400, and it requires two refills for 15 days of treatment. Additionally, insulin is required, so there will be a significant out-of-pocket expense.
In the meantime, your Medicare plan may cover your insulin pen, Tandem t.slim X2, Omnipod 5 insulin pump, or any other insulin device. But, if you are concerned about the price of insulin, don't worry – Medicare Part D will cover it. In fact, it will help you reduce your overall expenses by reducing the amount of money you spend on insulin. In addition, Medicare Part D can cover insulin pump supplies, such as test strips, lancets, and control solutions.
It is waterproof

The Omnipod system is a tubeless, tube-free insulin delivery device. Its insertion components and pumping mechanism are all waterproof. Here are some common questions about this device and how it can be used to control blood glucose levels. The Omnipod pump is also waterproof and can withstand 25 feet of water for an hour. It's a convenient option for people who are always on the go, or who want to minimize the need for a constant checkup.
The Pod is waterproof, with a waterproof IPX8 rating of up to 25 feet. The DASH PDM, however, is not waterproof. The Pod is waterproof and can withstand water up to 60 minutes, but the pump itself is not. You should never use an Omnipod pump while swimming, but it should be completely dry before recharging. Omnipod is a trademark of Insulet Corporation. It's worth checking out if this is the right pump for your needs.
If you have a problem with your pump, there are a few things to consider. First, is the pump itself waterproof? The Omnipod pump is waterproof, but the Pod controller and PDM are not. To clean the Pod, use a damp cloth and mild soap and water. Make sure you don't use harsh detergents or solvents as they may damage the casing or irritate the infusion site.
It integrates with Dexcom G6
The newest insulin pump from Omnipod combines a continuous glucose monitor and tubeless insulin pump. The pump is compatible with Dexcom G6 continuous glucose monitor, and its new smartAdjust technology makes it even smarter. The pump can be controlled by a mobile app downloaded on a compatible smartphone or by using the controller provided with your first prescription. SmartAdjust receives glucose trend data from your Dexcom CGM every five minutes and predicts your future glucose levels using a personalized glucose target. With this feature, the pump helps you avoid highs and lows in your blood sugar levels.
The pump is capable of communicating directly with the Dexcom G6. However, the pump has several limitations, including the fact that it doesn't talk directly to the CGM and doesn't automatically adjust insulin doses based on CGM data. Ryan's basal insulin needs change constantly as he goes through puberty. It's tiring to have to check the CGM for glucose levels all the time. The new Omnipod 5 is a welcome addition to the insulin pump market.
The Omnipod 5 automated insulin delivery system has been cleared by the FDA for use by people with type 1 diabetes. It is the first tubeless closed-loop system approved by the FDA. It also integrates with a Dexcom G6 continuous glucose monitoring device. With the Omnipod 5 system, you no longer need to worry about plastic tubing and can control your insulin using your smartphone. The device is designed to make glucose management easier and less stressful.
In April, Insulet and Dexcom announced partnerships to integrate the G6 with Omnipod Horizon Automated Insulin Delivery System. The new system will automatically adjust insulin dose based on the sensor values from your Dexcom G6 or Dexcom G7 CGM. The new system also has a CGM app to connect to your Omnipod insulin pump. This new system will be launched in early 2021.










